Chapter 1 General Introduction
1. Different ionized gaes
- H II regions: to probe the evolution of the elements and the star formation history of galaxies
- There are O- or early B-type stars (young, hot, luminous stars) with effective temperature of
. - H is ionized, part of the He is ionized.
- Typical density:
- Velocity of gas
, close to the isothermal sound speed. - Strong H I recombination lines, [N II] and [O II] collionally excited lines.
- Concentrated to the spiral arms for the Milky Way.
- There are O- or early B-type stars (young, hot, luminous stars) with effective temperature of
- Planetary nebulae: the outer remaining envelopes of dying stars
- There are dying stars with effective temperature of
. - More ionized than H II region
- Typical density:
- There are dying stars with effective temperature of
- Supernova remnants: to observe material from the burned-out deep interiors of supernovae
- Gas around active galactic nuclei: to study the central engines of galaxies
2. Spectra
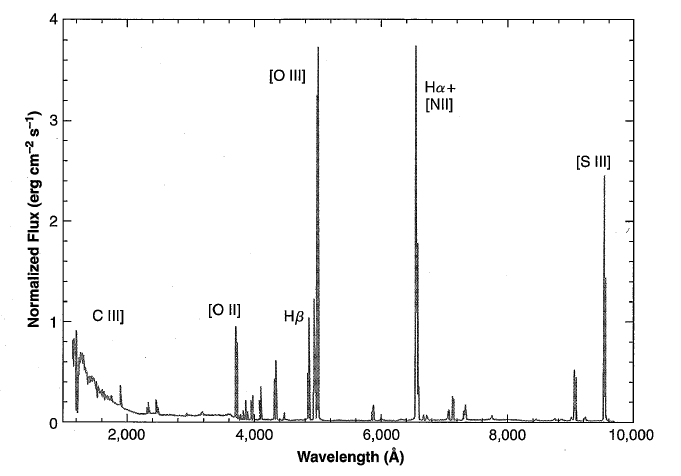
- Emission lines: dominated by forbidden lines like [O III]
, [NII] , and [S II] ; by permitted lines like H and H . More details are available in Figure 1.1. - Weak continuous spectra: consist of atomic and reflection components.
- Atomic continuum: chiefly by free-bound transitions, mainly in the Paschen continuum of H I at
, and the Balmer continuum at . - Reflection continua: starlight scattered by dust.
- Atomic continuum: chiefly by free-bound transitions, mainly in the Paschen continuum of H I at
- Continous spectrum is strong in the radio-frequency region.
3. Physical conditions
- The energy source: ultraviolet radiation from stars. The effective surface temperature of hot stars can be as high as
. - The main energy-input mechaism: the photoionization of H.
- The liberated photoelectrons collide with ions and hence excite the low energy levels of the ions.
- Although the probabilities of the radiative decay of the excited levels is small, the collisional de-excitation is even more inefficient as a consequence of low density (
). Therefore, almost every excitation leads to emission of a photon. - There is an equilibrium between the photoionization and recombination processes, which is called the ionization equilibrium.
- The recombination of a ion will form a excited atoms, such as H
give rise to excited atoms of H and leads to the emission of H I (the origin of the H I Balmer- and Paschen-line spectra). - Inelastic collisions of free electrons and ions converts kinetic energy into excitation energy, which is called the collisional excitation. The inverse process is called the collisional de-excitation. There are two ways to lose the excitation energy for a excited ions: (1) radiative decay; (2) collisional de-excitation.
- There are almost no lines for the ions that are fully ionized in the hot gas. Although a single nucleus can be excited, but the energy is too high, which is
. NuDat 3.0 gives the energy levels for the nuclei. NuDat 3.0 for Fe shows the energy levels for Fe. The energy of the first excited level for Fe is , which is too high to be excited in the hot gas. The hydrogen nucleus is even more stable.